146
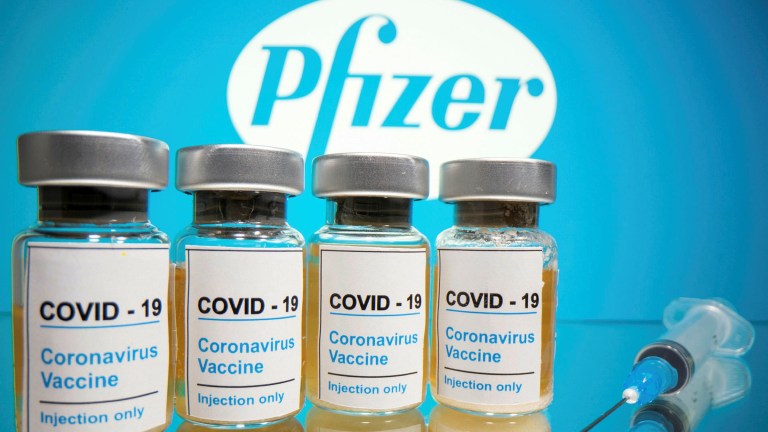
The National Agency for Food and Drug Administration and Control (NAFDAC) on Friday has approved Pfizer COVID-19 vaccine for use in Nigeria.
Nigeria had procured Oxford/AstraZeneca COVID-19 vaccine, which is being administered currently across the country to curtail the spread of coronavirus.
The Director-General of NAFDAC, Mojisola Adeyeye disclosed this in a press conference while addressing Journalists on Friday She however noted that the pfizer-Bio Tech vaccine was for emergency use only.
She added that the vaccine can now be stored between -15 to-25 equivalent to freezer temperature.